\( \DeclareMathOperator{\abs}{abs} \newcommand{\ensuremath}[1]{\mbox{$#1$}} \)
Atomic Orbital Plotting with GNU/Linux
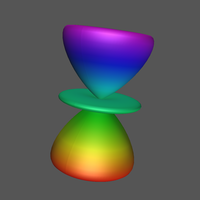
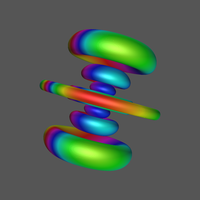

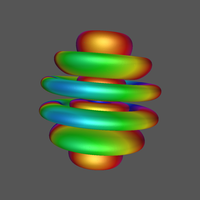
1 What is an atomic orbital (AO)?
The matter wave functions derive from the Schroedinger equation and are only approximations to the real behavior, and spatial location, of electrons. The Schroedinger equation does not account for either the "spin" angular momentum of the electron or relativistic effects. However, to obtain a basic, qualitative grasp of many atomic properties and behavior the atomic orbital picture arising from the Schroedinger equation is very useful.
We begin by providing a quick mathematical background of the Schroedinger equation (SE) and its solution as it is applied to a one-electron, hydrogen-like atom.
2 The Schroedinger Equation (Time Independent)
|
funmake
(
Ψ
,
[
x
,
y
,
z
]
)
$
− ħ ^ 2 / ( 2 · m ) · ( diff ( Ψ ( x , y , z ) , x , 2 ) + diff ( Ψ ( x , y , z ) , y , 2 ) + diff ( Ψ ( x , y , z ) , z , 2 ) ) + V ( x , y , z ) · Ψ ( x , y , z ) = E · Ψ ( x , y , z ) ; |
\[\operatorname{ }\operatorname{V}\left( x\operatorname{,}y\operatorname{,}z\right) \operatorname{\Psi }\left( x\operatorname{,}y\operatorname{,}z\right) -\frac{\left( \frac{{{∂}^{2}}}{∂ {{z}^{2}}} \operatorname{\Psi }\left( x\operatorname{,}y\operatorname{,}z\right) +\frac{{{∂}^{2}}}{∂ {{y}^{2}}} \operatorname{\Psi }\left( x\operatorname{,}y\operatorname{,}z\right) +\frac{{{∂}^{2}}}{∂ {{x}^{2}}} \operatorname{\Psi }\left( x\operatorname{,}y\operatorname{,}z\right) \right) {{\hbar }^{2}}}{2 m}=E \operatorname{\Psi }\left( x\operatorname{,}y\operatorname{,}z\right) \]
1) The SE is expressed in spherical coordinates, \( (r, \theta, \phi) \), which are most appropriate for an atomic system, where it becomes separable. For the case of a one-electron, hydrogen-like atom the potential energy is \( V(r) = e^{\frac{2}{r}} \), where e is the electron charge.
2) The solution, \( \Psi \), is therefore the product of the separated functions in the variables:
\( \Psi(r, \theta, \phi) = R(r) \cdot Y(\theta, \phi) \), i.e. a radial function and an angular function.
The quantum numbers, \( n, l, m \), which define an atomic state, arise from the fact that both the radial and angular functions are expressed, in part, as polynomials which can only exist with certain integral parameters. These parameters then become the quantum numbers. In fact, the angular solution, \( Y(\theta, \phi) \), is nothing more than the ordinary spherical harmonics.
More depth and details are here: http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/hydsch.html
2.1 The Radial Component of the SE Solution
| R_nl ( r ) : = ρ ^ ( 3 / 2 ) · sqrt ( ( n − l − 1 ) ! / ( 2 · n · ( ( n + l ) ! ) ) ) · exp ( − ρ · r / 2 ) · ( ρ · r ) ^ l · L ( ρ · r ) ; |
\[\operatorname{ }\operatorname{R\_ nl}(r)\operatorname{:=}{{\rho }^{\frac{3}{2}}} \sqrt{\frac{\left( n-l-1\right) \operatorname{!}}{2 n\, \left( n+l\right) \operatorname{!}}} \operatorname{exp}\left( \frac{\left( -\rho \right) r}{2}\right) {{\left( \rho r\right) }^{l}} \operatorname{L}\left( \rho r\right) \]
\( L(\rho \cdot r) \)is the associated (generalized) Laguerre polynomial and depends on the quantum numbers \( n, l \).
Note that \( R(r) \) is a real-valued function in \( r \).
2.2 The Angular Component of the SE Solution
| Y_ml ( θ , φ ) : = %i ^ ( m + abs ( m ) ) · sqrt ( ( 2 · l + 1 ) / ( 4 · %pi ) ) · sqrt ( ( l − abs ( m ) ! ) / ( l + abs ( m ) ! ) ) · P · ( cos ( θ ) ) · %e ^ ( %i · m · φ ) ; |
\[\operatorname{ }\operatorname{Y\_ ml}\left( \theta \operatorname{,}\phi \right) \operatorname{:=}{{i}^{m+\left| m\right| }} \sqrt{\frac{2 l+1}{4 \ensuremath{\pi} }} \sqrt{\frac{l-\left| m\right| \operatorname{!}}{l+\left| m\right| \operatorname{!}}} P \cos{\left( \theta \right) } {{e}^{i m \phi }}\]
Note that \( Y(\theta, \phi) \) is a complex-valued function in \( \theta, \phi \).
3 The Background on Plotting the Atomic Orbitals
Geomview with StageTools is then used to create a plot and optional animations to allow a fuller appreciation of the AO "shapes."
Keep in mind that AO plots are actually 4-dimensional, that is the value of the wave function depends on 3 spatial coordinates: \( \Psi = \Psi(r, \theta, \phi) \). Such functions cannot be plotted in 3-D space. Therefore the AO can only be visualized by taking a 3-D "slice" at a particular value of the probability density.
We shall consider first the angular probability density which, because it depends only on two variables, \( \theta \) and \( \phi \), can be directly visualized. However this plot does not provide a true indication of orbital shape because the angular density is modulated by the radial density function which can contain "nodes" or points where the wave function (and concommitant probability) is zero.
Secondly, the radial density function is presented as a 2-D plot since only a single variable, \( r \), is involved.
Lastly, the two plots are combined (multiplied) to reveal the true orbital picture. The initial plot will consist of contours at select values of probability density. This is accomplished by eliminating the \( \phi \) variable due to spherical symmetry. Then, at a single value of probability density a surface of rotation will be created to depict the "true" orbital shape (but see the caveat below).
Unfortunately, the production of such countour plots requires using implicit function methods which are beyond the capabilities of StageTools. Rather I will use the Linux version of Mathematica to produce the contour plots and then export them to an OFF format which can then be rendered by Geomview. All other plots, however, are produced by either wxMaxima or the StageTools module.
It is important to note that most textbooks on atomic orbitals, and especially chemistry texts, do not plot the actual complex-valued solutions of the SE. Rather, real-valued solutions are obtained through linear combinations of the complex wave functions.
We shall not follow this common approach. Although such real-valued functions are perfectly valid solutions to the SE they contain "superpositions" and cannot in many cases be given a definite value of the m quantum number. To ensure that each AO picture is based on a definite set of quantum numbers we will use only the complex-valued solutions of the SE.
4 Plotting the Atomic Orbitals
It should be noted that the complex portion of the angular function, \( e^{i m φ} \), when squared by multiplying by the complex conjugate, equals \( 1 \) for any \( m \). As a consequence, it will always be omitted from the following calculations. The exception is when the complex phase information is required for coloring the plots.
4.1 Quantum Numbers n=2, l=0, and m=0.
A lot of background information will be provided with this first example that will be omitted from subsequent examples.
| kill ( all ) $ load ( orthopoly ) $ load ( draw ) $ |
|
set_draw_defaults
(
xu_grid
=
100
,
yv_grid
=
100
,
nticks
=
100
,
background_color
=
light_yellow
,
ip_grid = [ 100 , 100 ] , ip_grid_in = [ 100 , 100 ] , xlabel = "Bohr Radius Units" ) $ |
|
n
:
2
$
l
:
0
$
m
:
0
$
coeff : %i ^ ( m + abs ( m ) ) · sqrt ( ( ( l − abs ( m ) ) ! ) / ( ( l + abs ( m ) ) ! ) ) · sqrt ( ( 2 · l + 1 ) / 4 / %pi ) $ P_lm : expand ( assoc_legendre_p ( l , abs ( m ) , cos ( θ ) ) ) $ define ( aprob ( θ , φ ) , ( coeff · P_lm ) ^ 2 ) ; /* calculate radial probability */ a : n − l − 1 $ b : 2 · l + 1 $ ρ : 4 / n $ L : expand ( sum ( ( ( a + b ) ! ) / ( ( k + b ) ! ) / ( ( a − k ) ! ) / ( k ! ) · ( − r · ρ ) ^ k , k , 0 , a ) · ρ ^ l · r ^ l ) $ Rcoeff : ρ ^ ( 3 / 2 ) · sqrt ( ( ( n − l − 1 ) ! ) / ( 2 · n ) / ( ( n + l ) ! ) ) · exp ( − ρ · r / 2 ) $ define ( rprob ( r ) , ( L · Rcoeff ) ^ 2 ) ; |
\[\operatorname{ }\operatorname{aprob}\left( \theta \operatorname{,}\phi \right) \operatorname{:=}\frac{1}{4 \pi }\]
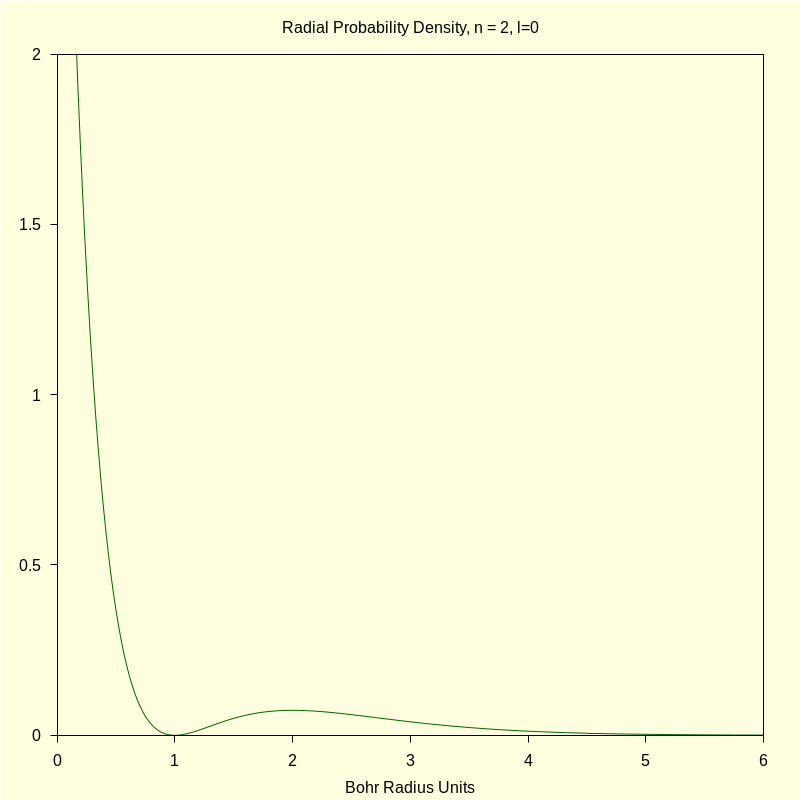
\[\operatorname{ }\operatorname{rprob}(r)\operatorname{:=}{{\left( 2-2 r\right) }^{2}} {{e}^{-2 r}}\]
|
/* check for normalization */
' integrate ( rprob ( r ) · r ^ 2 , r , 0 , inf ) ; integrate ( rprob ( r ) · r ^ 2 , r , 0 , inf ) ; inner_int : ' integrate ( aprob ( θ , φ ) · sin ( θ ) , θ , 0 , %pi ) $ ' ( integrate ( ' ' inner_int , φ , 0 , 2 · %pi ) ) ; integrate ( integrate ( aprob ( θ , φ ) · sin ( θ ) , θ , 0 , %pi ) , φ , 0 , 2 · %pi ) ; |
\[\operatorname{ }\int_{0}^{\infty }{\left. {{\left( 2-2 r\right) }^{2}} {{r}^{2}} {{e}^{-2 r}}dr\right.}\]
\[\operatorname{ }1\]
\[\operatorname{ }\int_{0}^{2 \ensuremath{\pi} }{\left. \frac{\int_{0}^{\ensuremath{\pi} }{\left. \sin{\left( \theta \right) }d\theta \right.}}{4 \ensuremath{\pi} }d\phi \right.}\]
\[\operatorname{ }1\]
4.1.1 Angular Probability Density
Click Image for Video
4.1.2 Radial Probability Density
|
wxdraw2d
(
proportional_axes
=
none
,
color
=
dark_green
,
explicit
(
rprob
,
r
,
0
,
6
)
,
title
=
"Radial Probability Density, n = 2"
,
ytics = true , xrange = [ 0 , 6 ] , yrange = [ 0 , 2 ] ) $ |
\[\operatorname{ }\]
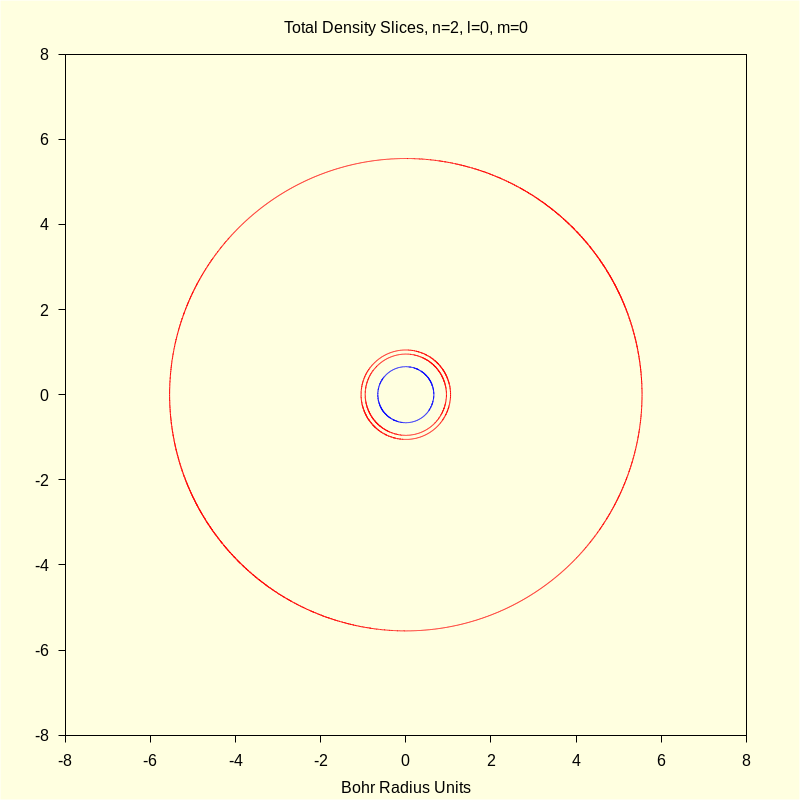
4.1.3 Total Density "Slices"
|
p1
:
1e-2
$
p2
:
1e-4
$
wxdraw2d ( color = blue , proportional_axes = xy , implicit ( rprob ( sqrt ( x ^ 2 + y ^ 2 ) ) · aprob ( atan2 ( x , y ) , 0 ) − p1 , x , − 8 , 8 , y , − 8 , 8 ) , color = red , implicit ( rprob ( sqrt ( x ^ 2 + y ^ 2 ) ) · aprob ( atan2 ( x , y ) , 0 ) − p2 , x , − 8 , 8 , y , − 8 , 8 ) ) $ |
4.1.4 Total Density Contour Surface
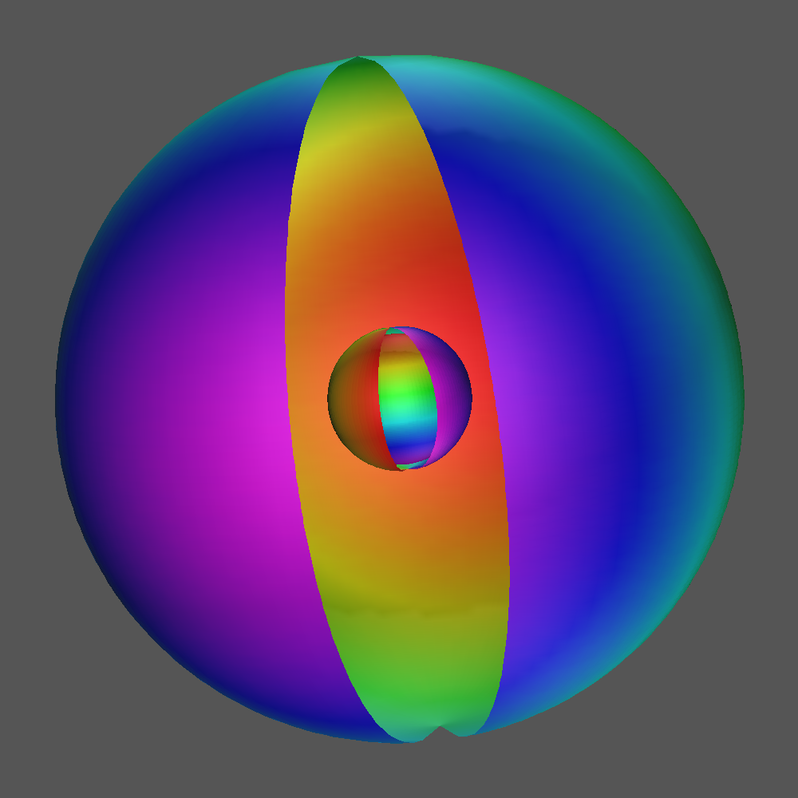
Click Image for Video
4.2 Quantum Numbers n=6, l=3, and m=1.
This orbital has the physical designation \( \Psi\_631 \).
| kill ( all ) $ |
|
n
:
6
$
l
:
3
$
m
:
1
$
coeff : %i ^ ( m + abs ( m ) ) · sqrt ( ( ( l − abs ( m ) ) ! ) / ( ( l + abs ( m ) ) ! ) ) · sqrt ( ( 2 · l + 1 ) / 4 / %pi ) $ P_lm : expand ( assoc_legendre_p ( l , abs ( m ) , cos ( θ ) ) ) $ define ( aprob ( θ , φ ) , ( coeff · P_lm ) ^ 2 ) ; /* calculate radial probability */ a : n − l − 1 $ b : 2 · l + 1 $ ρ : 4 / n $ L : expand ( sum ( ( ( a + b ) ! ) / ( ( k + b ) ! ) / ( ( a − k ) ! ) / ( k ! ) · ( − r · ρ ) ^ k , k , 0 , a ) · ρ ^ l · r ^ l ) $ Rcoeff : ρ ^ ( 3 / 2 ) · sqrt ( ( ( n − l − 1 ) ! ) / ( 2 · n ) / ( ( n + l ) ! ) ) · exp ( − ρ · r / 2 ) $ define ( rprob ( r ) , ( L · Rcoeff ) ^ 2 ) ; |
\[\operatorname{ }\operatorname{aprob}\left( \theta \operatorname{,}\phi \right) \operatorname{:=}\frac{7 {{\left( \frac{3 \sqrt{1-{{\cos{\left( \theta \right) }}^{2}}}}{2}-\frac{15 {{\cos{\left( \theta \right) }}^{2}} \sqrt{1-{{\cos{\left( \theta \right) }}^{2}}}}{2}\right) }^{2}}}{48 \pi }\]
\[\operatorname{ }\operatorname{rprob}(r)\operatorname{:=}\frac{{{\left( \frac{16 {{r}^{5}}}{243}-\frac{16 {{r}^{4}}}{9}+\frac{32 {{r}^{3}}}{3}\right) }^{2}} {{e}^{-\frac{2 r}{3}}}}{7348320}\]
|
/* check for normalization */
' integrate ( rprob ( r ) · r ^ 2 , r , 0 , inf ) ; integrate ( rprob ( r ) · r ^ 2 , r , 0 , inf ) ; inner_int : ' integrate ( aprob ( θ , φ ) · sin ( θ ) , θ , 0 , %pi ) $ ' ( integrate ( ' ' inner_int , φ , 0 , 2 · %pi ) ) ; integrate ( integrate ( aprob ( θ , φ ) · sin ( θ ) , θ , 0 , %pi ) , φ , 0 , 2 · %pi ) ; |
\[\operatorname{ }\frac{\int_{0}^{\infty }{\left. {{r}^{2}} {{\left( \frac{16 {{r}^{5}}}{243}-\frac{16 {{r}^{4}}}{9}+\frac{32 {{r}^{3}}}{3}\right) }^{2}} {{e}^{-\frac{2 r}{3}}}dr\right.}}{7348320}\]
\[\operatorname{ }1\]
\[\operatorname{ }\int_{0}^{2 \ensuremath{\pi} }{\left. \frac{7 \int_{0}^{\ensuremath{\pi} }{\left. {{\left( \frac{3 \sqrt{1-{{\cos{\left( \theta \right) }}^{2}}}}{2}-\frac{15 {{\cos{\left( \theta \right) }}^{2}} \sqrt{1-{{\cos{\left( \theta \right) }}^{2}}}}{2}\right) }^{2}} \sin{\left( \theta \right) }d\theta \right.}}{48 \ensuremath{\pi} }d\phi \right.}\]
\[\operatorname{ }1\]
4.2.1 Angular Probability Density
Click Image for Video
4.2.2 Radial Probability Density
| wxdraw2d ( proportional_axes = none , color = dark_green , explicit ( rprob , r , 0 , 50 ) , title = "Radial Probability Density, n = 6" ) $ |
\[\operatorname{ }\]
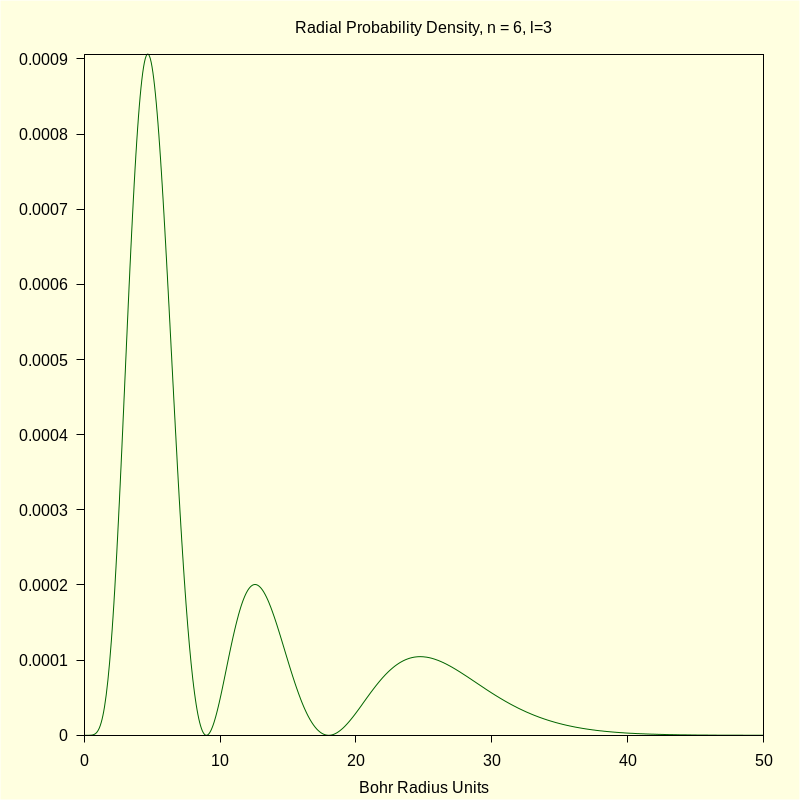
4.2.3 Total Density "Slices"
|
p1
:
1e-6
$
p2
:
1e-5
$
wxdraw2d ( color = blue , implicit ( rprob ( sqrt ( x ^ 2 + y ^ 2 ) ) · aprob ( atan2 ( x , y ) , 0 ) − p1 , x , − 50 , 50 , y , − 50 , 50 ) , color = red , implicit ( rprob ( sqrt ( x ^ 2 + y ^ 2 ) ) · aprob ( atan2 ( x , y ) , 0 ) − p2 , x , − 50 , 50 , y , − 50 , 50 ) ) ; |
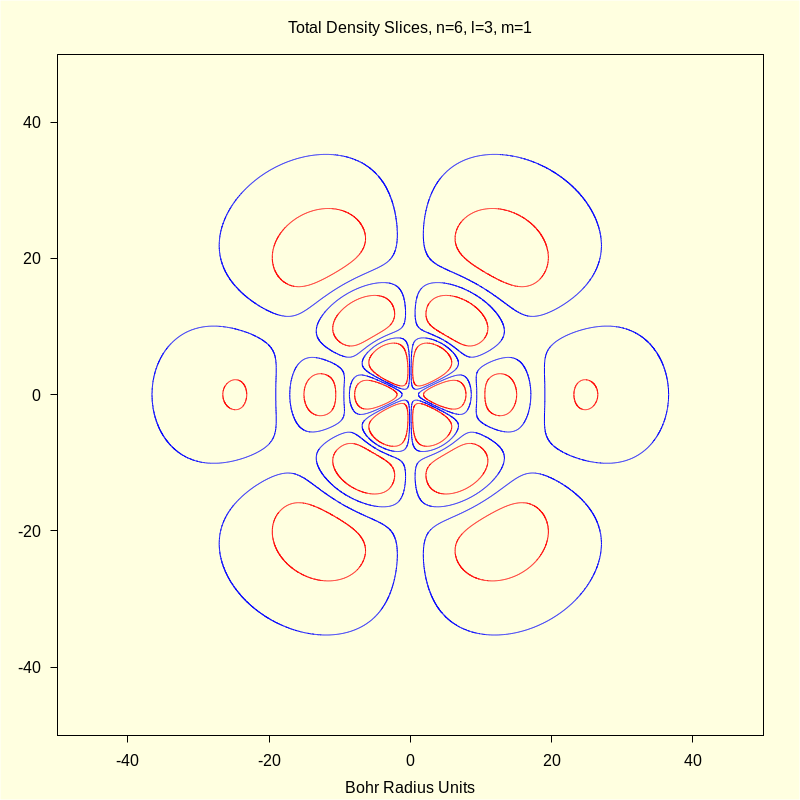
\[\operatorname{ }\]
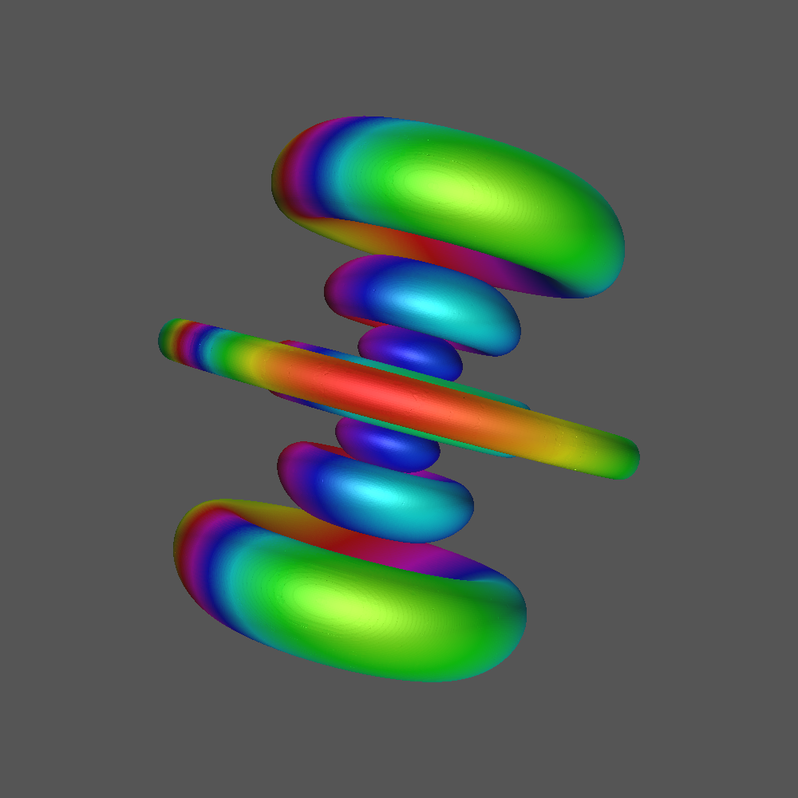
Click Image for Video
4.3 Quantum Numbers n=8, l=6, and m=1.
This orbital, or linear combinations thereof, is sometimes called the i orbital but the precise designation is \( \Psi\_861 \).
|
n
:
8
$
l
:
6
$
m
:
1
$
/* calculate angular probability */ Acoeff : %i ^ ( m + abs ( m ) ) · sqrt ( ( ( l − abs ( m ) ) ! ) / ( ( l + abs ( m ) ) ! ) ) · sqrt ( ( 2 · l + 1 ) / 4 / %pi ) $ P_lm : expand ( assoc_legendre_p ( l , abs ( m ) , cos ( θ ) ) ) $ define ( aprob ( θ , φ ) , trigreduce ( ( Acoeff · P_lm ) ^ 2 ) ) ; /* calculate radial probability */ a : n − l − 1 $ b : 2 · l + 1 $ ρ : 4 / n $ L : expand ( sum ( ( ( a + b ) ! ) / ( ( k + b ) ! ) / ( ( a − k ) ! ) / ( k ! ) · ( − r · ρ ) ^ k , k , 0 , a ) · ρ ^ l · r ^ l ) $ Rcoeff : ρ ^ ( 3 / 2 ) · sqrt ( ( ( n − l − 1 ) ! ) / ( 2 · n ) / ( ( n + l ) ! ) ) · exp ( − ρ · r / 2 ) $ define ( rprob ( r ) , ( L · Rcoeff ) ^ 2 ) ; |
\[\operatorname{ }\operatorname{aprob}\left( \theta \operatorname{,}\phi \right) \operatorname{:=}\frac{-297297 \cos{\left( 12 \theta \right) }-216216 \cos{\left( 10 \theta \right) }-129402 \cos{\left( 8 \theta \right) }-32760 \cos{\left( 6 \theta \right) }+83265 \cos{\left( 4 \theta \right) }+248976 \cos{\left( 2 \theta \right) }+343434}{1048576 \ensuremath{\pi} }\]
\[\operatorname{ }\operatorname{rprob}(r)\operatorname{:=}\frac{{{\left( \frac{7 {{r}^{6}}}{32}-\frac{{{r}^{7}}}{128}\right) }^{2}} {{e}^{-\frac{r}{2}}}}{11158821273600}\]
|
/* check for normalization */
' integrate ( rprob ( r ) · r ^ 2 , r , 0 , inf ) ; integrate ( rprob ( r ) · r ^ 2 , r , 0 , inf ) ; ' ( integrate ( ' integrate ( aprob ( θ , φ ) · sin ( θ ) , θ , 0 , %pi ) , φ , 0 , 2 · %pi ) ) ; integrate ( integrate ( aprob ( θ , φ ) · sin ( θ ) , θ , 0 , %pi ) , φ , 0 , 2 · %pi ) ; |
\[\operatorname{ }\frac{\int_{0}^{\infty }{\left. {{r}^{2}} {{\left( \frac{7 {{r}^{6}}}{32}-\frac{{{r}^{7}}}{128}\right) }^{2}} {{e}^{-\frac{r}{2}}}dr\right.}}{11158821273600}\]
\[\operatorname{ }1\]
\[\operatorname{ }\int_{0}^{2 \ensuremath{\pi} }{\left. \int_{0}^{\ensuremath{\pi} }{\left. \sin{\left( \theta \right) } \operatorname{aprob}\left( \theta \operatorname{,}\phi \right) d\theta \right.}d\phi \right.}\]
\[\operatorname{ }1\]
4.3.1 Angular Probability Density
To fully appreciate the shape of this angular density be sure to view the MP4 format video by clicking the image or link.

Click Image for Video
4.3.2 Radial Probability Density
|
wxdraw2d
(
proportional_axes
=
none
,
color
=
dark_green
,
explicit
(
rprob
,
r
,
0
,
100
)
,
title = "Radial Density, n=8, l=6" , xlabel = "Bohr Radius Units" ) $ |
\[\operatorname{ }\]
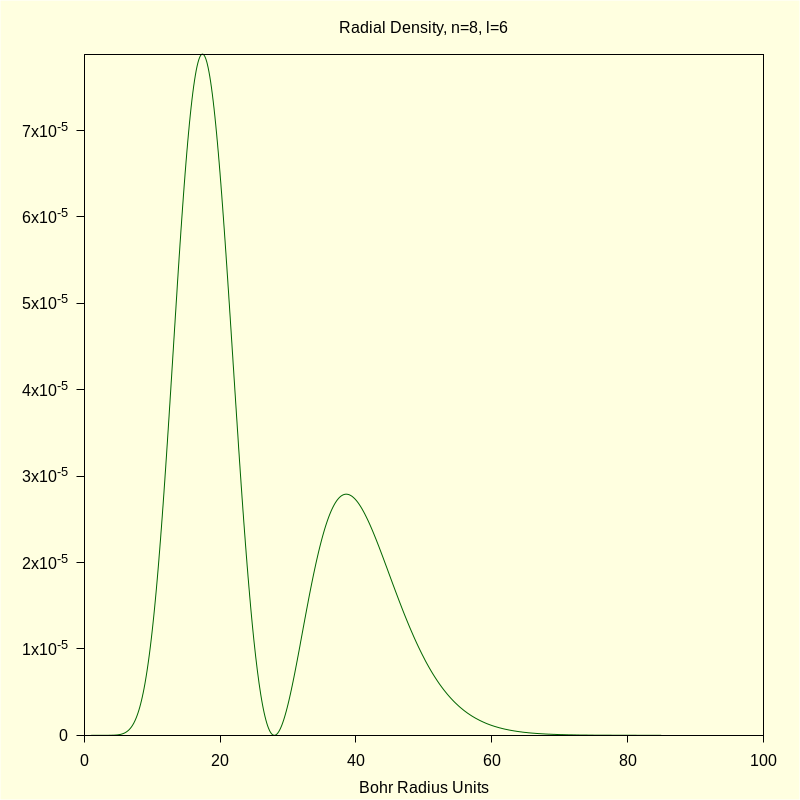
4.3.3 Total Density "Slices"
|
p1
:
1e-6
$
p2
:
4e-6
$
wxdraw2d ( color = blue , implicit ( rprob ( sqrt ( x ^ 2 + y ^ 2 ) ) · aprob ( atan2 ( x , y ) , 0 ) − p1 , x , − 80 , 80 , y , − 80 , 80 ) , color = red , implicit ( rprob ( sqrt ( x ^ 2 + y ^ 2 ) ) · aprob ( atan2 ( x , y ) , 0 ) − p2 , x , − 50 , 50 , y , − 50 , 50 ) , title = "Total Density Slices, n=8, l=6, m=1" , xlabel = "Bohr Radius Units" ) $ |
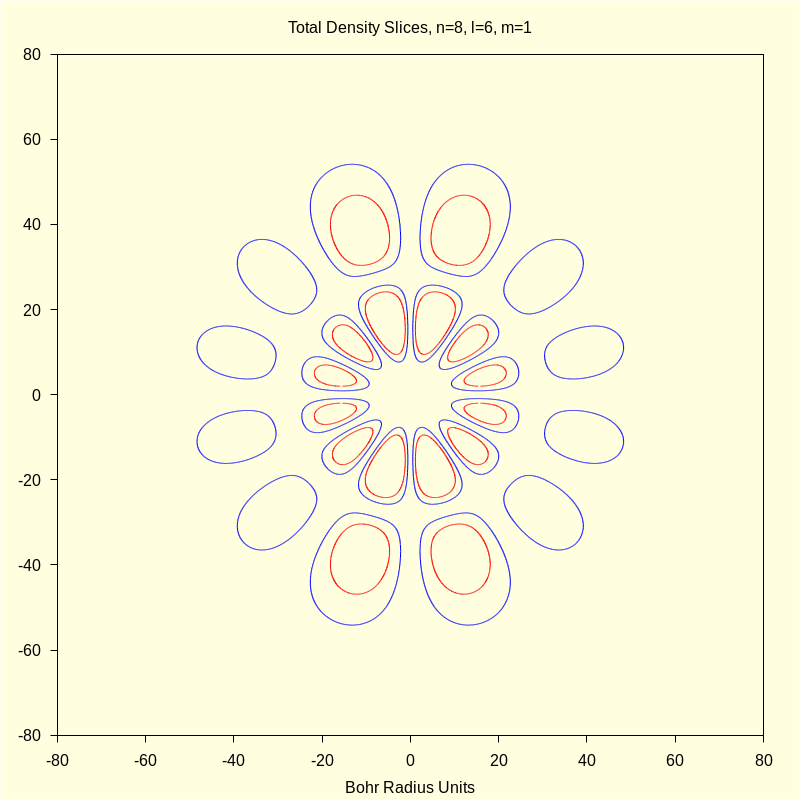
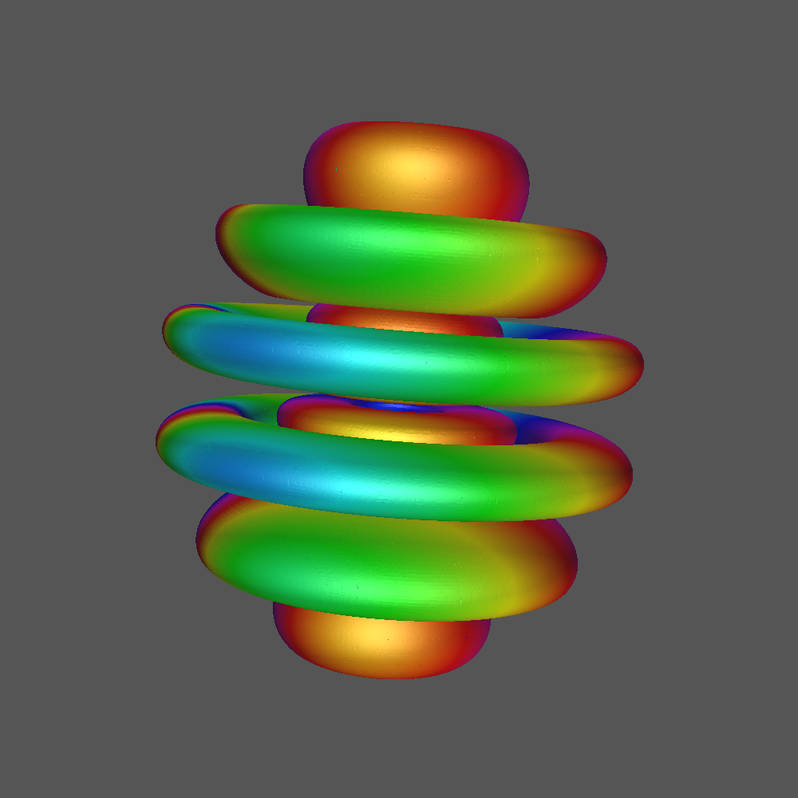
Click Image for Video
5 Epilogue
As a supplement, the graphic utilities of Geomview can produce stunning output and, coupled with StageTools and ffmpeg, AO videos can be realized.
As always, the interested reader is encouraged to explore these, and other, GNU/Linux tools.
Created with
wxMaxima.
Modified and embedded by L.A.P.